Capsugel® DBcaps® capsules
Double blind. Zero bias.

Features
Benefits
Wide size range available supporting over-encapsulation
of 90% of marketed solid oral dosage forms
Speed to clinic, no change in dosage form properties,
easy blinding process
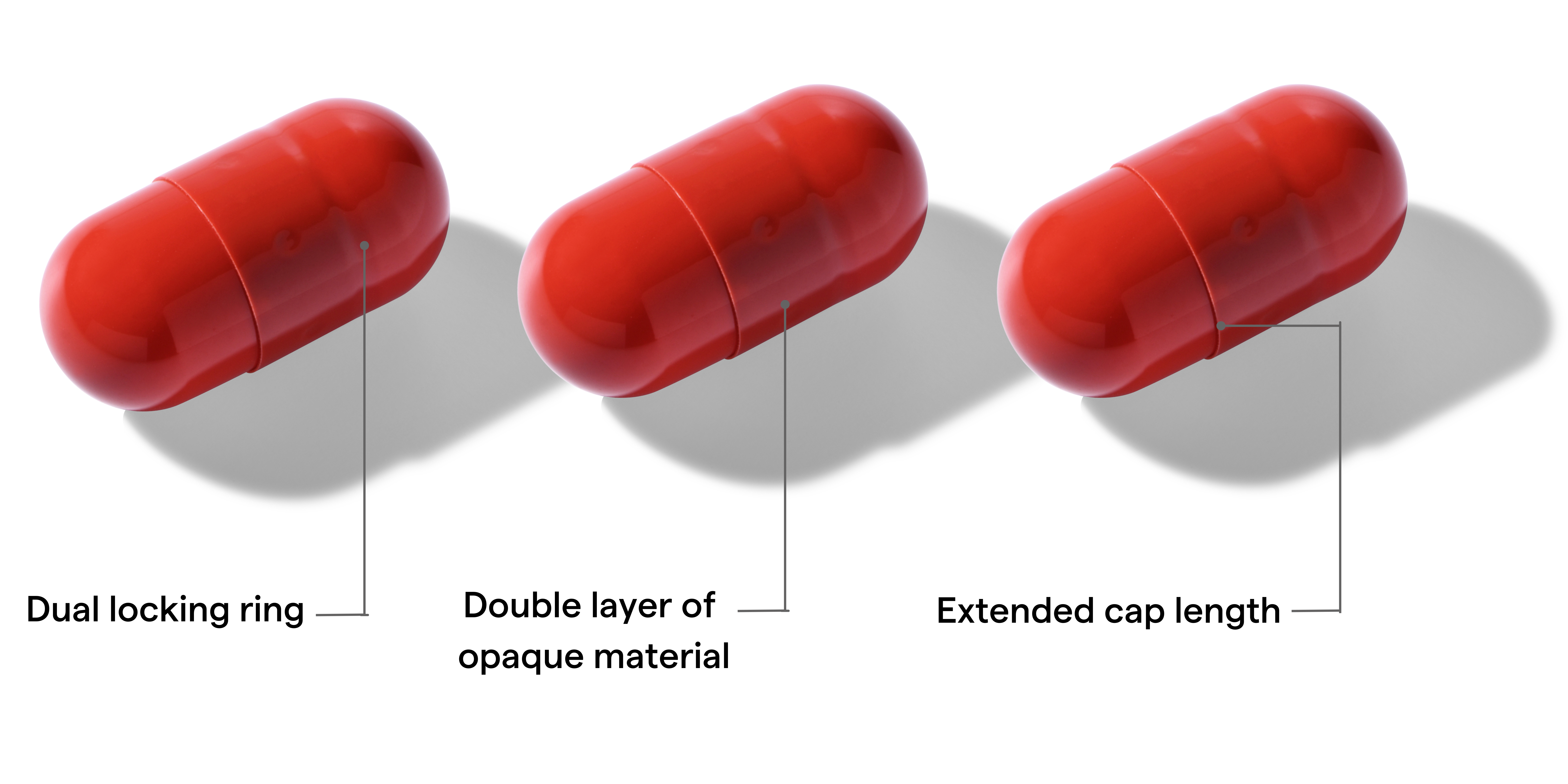
Design with double opaque layer of cap over body
Difficult for patient to open
Avoid “breaking the blind” through visual recognition
Available in gelatin and HPMC
Ability to adapt your study to the blinding form characteristics
(hygroscopicity, prone to cross-linking)
Can be used on a wide choice of capsule filling machines – brands and outputs
Easy blinding process, speed to clinic
Compact size
Easier to swallow than a standard capsule
Specific design with limited visible part of the body
Differentiated outlook for the dosage form
Design is key
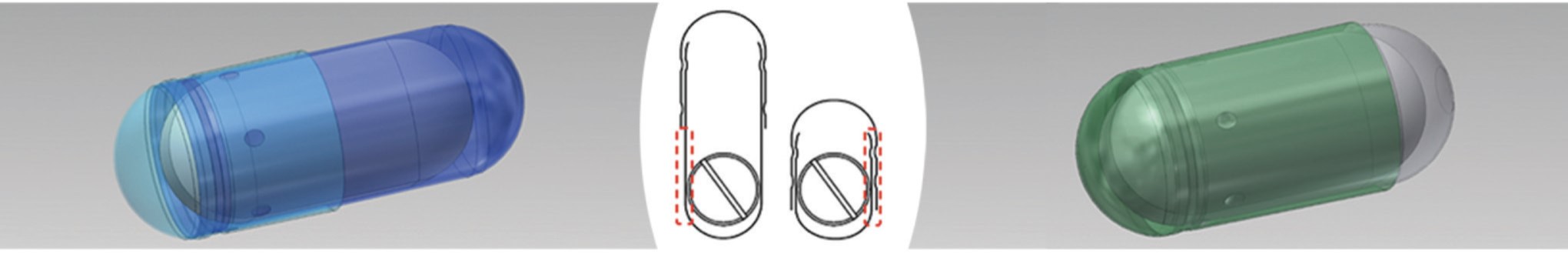
Standard capsule design
Risks for “breaking the blind” liked to single
layer of material on body in closed position
Capsugel® DBcaps® capsule design
Unique design of cap on body in closed position
ensures more opacity and reinforces the blind
Available in different formats
DBcaps® capsules are shipped in fiber-free, corrugated polypropylene containers and will arrive in a sturdy, recyclable box that is anti-static incorporated.
This leading-edge pharmaceutical practice allows packaging (pallets or box) entry into cGMP environments.

DBcaps® capsules are also available in a convenient CapsuleCaddy™ Container - in the most used opaque color, perfect for clinical trial batches and R&D projects.

Not sure about the size you need? Request a DBcaps® size kit to select the right capsule size for your next clinical trial
Contact us:
Review and follow all product safety instructions. All information in this presentation corresponds to Lonza’s knowledge on the subject at the date
of publication, but Lonza makes no warranty as to its accuracy or completeness and Lonza assumes no obligation to update it. All information in this presentation is intended for use by recipients experienced and knowledgeable in the field, who are capable of and responsible for independently determining the suitability and to ensure their compliance with applicable law. Proper use of this information is the sole responsibility of the recipient. Republication of this information or related statements is prohibited. Information provided in this presentation by Lonza is not intended and should not be construed as a license to operate under or a recommendation to infringe any patent or other intellectual property right. All trademarks belong to Lonza or its affiliates or to their respective third party owners and are only being used for informational purposes. Third party copyrights are used under license. © 2024 Lonza. All rights reserved.
Legal Disclaimer | Privacy Policy | ©2026 Lonza. All Rights Reserved.